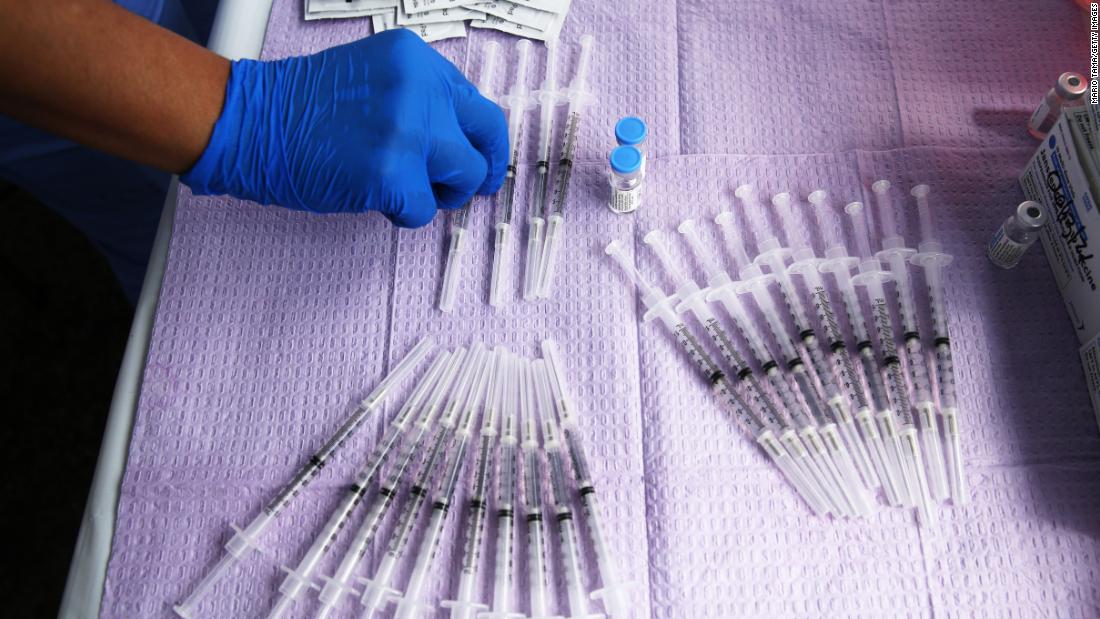
FDA report details flaws at Emergent facility making Johnson & Johnson Covid-19 vaccine
CNN
The US Food and Drug Administration said Wednesday that new production of Johnson & Johnson's Covid-19 vaccine remains paused at the Emergent BioSolutions facility where millions of potential doses were contaminated, and the agency and the company are working through a list of potential quality issues.
The FDA's inspection of Emergent's Bayview facility in Baltimore ended Tuesday and a newly released document details issues that could affect quality during manufacturing -- including incomplete investigations into cross-contamination, written procedures that weren't followed, poorly maintained facilities and a lack of employee training.
Oregon authorities are investigating a shooting by a Border Patrol agent in Portland that wounded two people federal authorities say are tied to a violent international gang – an incident that renewed questions about the Trump administration’s handling of its immigration crackdown in the city and across the US.

Mutual distrust between federal and state authorities derailed plans for a joint FBI and state criminal investigation into Wednesday’s shooting of a Minneapolis woman by an ICE officer, leading to the highly unusual move by the Justice Department to block state investigators from participating in the probe.

Vice President JD Vance’s claim Thursday that an Immigration and Customs Enforcement officer who fatally shot Renee Nicole Good in Minneapolis is “protected by absolute immunity” drew immediate pushback from experts who said the legal landscape around a potential prosecution is far more complicated.










