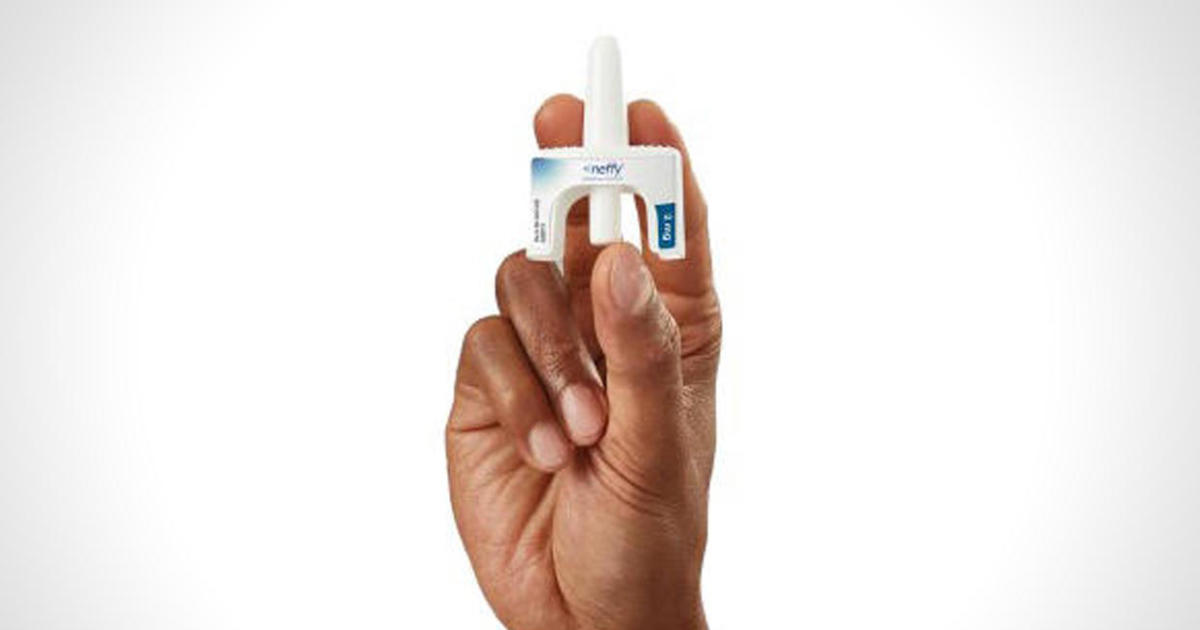
FDA rejects neffy epinephrine nasal spray for severe allergic reactions pending further trial data
CBSN
The Food and Drug Administration declined on Tuesday to approve neffy, an epinephrine nasal spray from drugmaker ARS Pharmaceuticals, keeping the first needle-free option for Americans to treat severe allergic reactions off the market pending more trial data.
ARS had expected the FDA to approve neffy for use in adults and children who weigh more than 30 kilograms, or around 66 pounds. The spray would have required a prescription, similar to EpiPens and other epinephrine injections that are currently used to treat anaphylaxis.
Epinephrine is crucial in an emergency to treat potentially life-threatening allergic reactions. The new spray, if eventually approved, would provide a welcome alternative for many families of children with severe allergies who'd rather avoid needles.
