Using bacteriophages to combat antimicrobial resistance Premium
The Hindu
Discover the potential solution to antibiotic resistance with bacteriophages, a natural alternative to combat bacterial infections effectively.
If one has a urinary tract infection, for instance, the pathology lab will identify the bacterium to be, say, Escherichia coli. It will also determine the pathogen’s sensitivity to over a dozen antibiotics. It is fine if the bacterium is sensitive to many or all of the drugs. The nightmare scenario is when it is resistant to all of them.
Increasingly, antibiotics don’t work because the bacteria have developed resistance. It is estimated that globally about five million people are dying of conditions related to antimicrobial resistance (AMR) each year. This may double by 2050. It is a silent pandemic.
What is the solution? Largely, pharmaceutical companies have lost interest in developing new antibiotics. Whereas a drug for cancer is used for a long time, antibiotics are given for just a few days. Also, due to the problem of AMR, new antibiotics are used as sparingly as possible to prevent the development of resistance. Therefore there is no financial incentive for companies to work on new antibiotics. There is some drug development happening but probably not enough to address the AMR problem.
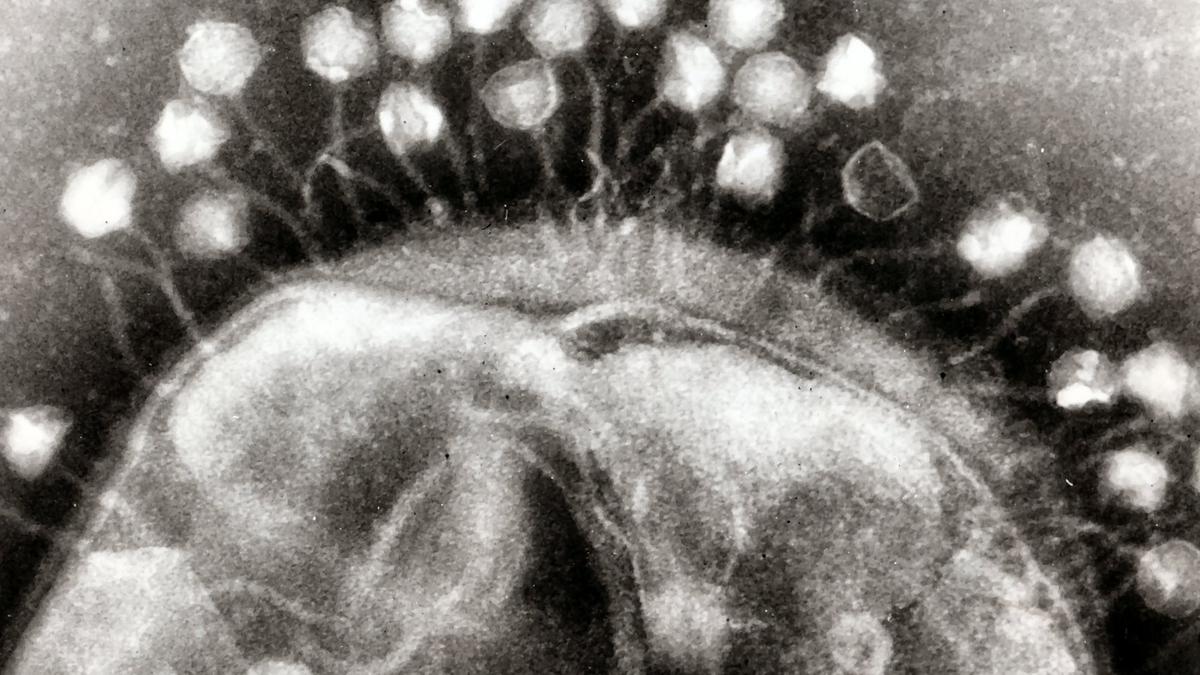
Bacteriophages are ‘good viruses’ that naturally prey on bacteria. They are all around us, in the water, in the soil, in our gut, on our skin, etc. There are believed to be 10-times as many phages as bacteria on the earth.
Phages were beginning to be used against bacterial infections about a century ago, but antibiotics superseded them once they were discovered. Unlike an antibiotic, which may be able to kill many species of bacteria, phages may only kill a few strains of a particular bacterium. Therefore only countries in the Soviet bloc, cut off from the antibiotics, continued to use them. An institute in Tbilisi, Georgia, with over 100 years of experience, is famous for its phage expertise. Due to AMR, the rest of the world is now rediscovering phages and relevant research is ongoing in many countries.
Phages have been used for burns, foot ulcers, gut infections, respiratory infections, urinary tract infections, etc. There are two main strategies that have been used. One, isolate the bacteria from the infected tissue, check which phage works against it in the lab, grow more of that phage and administer it to the patient. These phages may come from a phage bank of one’s own or in very serious cases one may even ask phage banks elsewhere in the world for help. These are natural phages. Then there are genetically engineered phages, which have been modified in the lab to, say, expand the variety of bacteria they can kill.
To the extent that phages are being used as drugs, they have a unique feature. Bacteria can evolve to be resistant to an antibiotic; likewise, bacteria can evolve to be resistant to a phage. The unique part is that the phages, too, can evolve to avoid the bacterial resistance. The drug is not a constant but an evolving entity. This is therefore a headache for the regulators, since no drug has ever been approved that evolves. Further, since phages are very specific to bacteria, one phage will not work against a large fraction of, say, foot ulcers, as happens with an antibiotic (until we have to consider AMR). So it is also challenging to conduct randomised controlled trials when the drug needed for each patient may be different.

How do you create a Christmas tree with crochet? Take notes from crochet artist Sheena Pereira, who co-founded Goa-based Crochet Collective with crocheter Sharmila Majumdar in 2025. Their artwork takes centre stage at the Where We Gather exhibit, which is part of Festivals of Goa, an ongoing exhibition hosted by the Museum of Goa. The collective’s multi-hued, 18-foot crochet Christmas tree has been put together by 25 women from across the State. “I’ve always thought of doing an installation with crochet. So, we thought of doing something throughout the year that would culminate at the year end; something that would resonate with Christmas message — peace, hope, joy, love,” explains Sheena.

Max Born made many contributions to quantum theory. This said, he was awarded the Nobel Prize for physics in 1954 for establishing the statistical interpretation of the ____________. Fill in the blank with the name of an object central to quantum theory but whose exact nature is still not fully understood.











