U.S. CDC backs COVID-19 shots for kids under 5. Here’s what to know
Global News
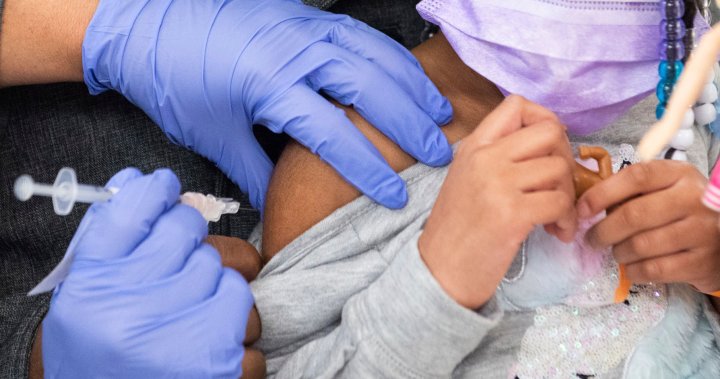
As the U.S. begins to roll out vaccines for kids as young as 6 months, around 18 million kids in the country will be eligible for COVID-19 vaccination.
U.S. health advisers on Saturday recommended COVID-19 vaccines for infants, toddlers and preschoolers — the last group without the shots.
The advisers to the Centers for Disease Control and Prevention (CDC) unanimously decided that coronavirus vaccines should be opened to children as young as 6 months. The final signoff was expected later in the day from CDC Director Dr. Rochelle Walensky.
While the Food and Drug Administration (FDA) OKs vaccines, it’s the CDC that decides who should get them.
The government has been gearing up for the start of the shots early next week, with millions of doses ordered for distribution to doctors, hospitals and community health clinics around the country.
Roughly 18 million kids will be eligible, but it remains to be seen how many will ultimately get the vaccines. Less than a third of children ages 5 to 11 have done so since vaccination opened up to them last November.
Here are some things to know:
Two brands — Pfizer and Moderna — got the green light Friday from the FDA. The vaccines use the same technology but are being offered at different dose sizes and number of shots for the youngest kids.
Pfizer’s vaccine is for 6 months through 4 years. The dose is one-tenth of the adult dose, and three shots are needed. The first two are given three weeks apart, and the last at least two months later.

Iran was largely cut off from the outside world on Friday after authorities blacked out the internet to curb growing unrest, as video showed buildings and vehicles ablaze in anti-government protests raging through the streets of several cities. In a televised address, Supreme Leader Ayatollah Ali Khamenei vowed not to back down, accusing demonstrators of acting on behalf of...












