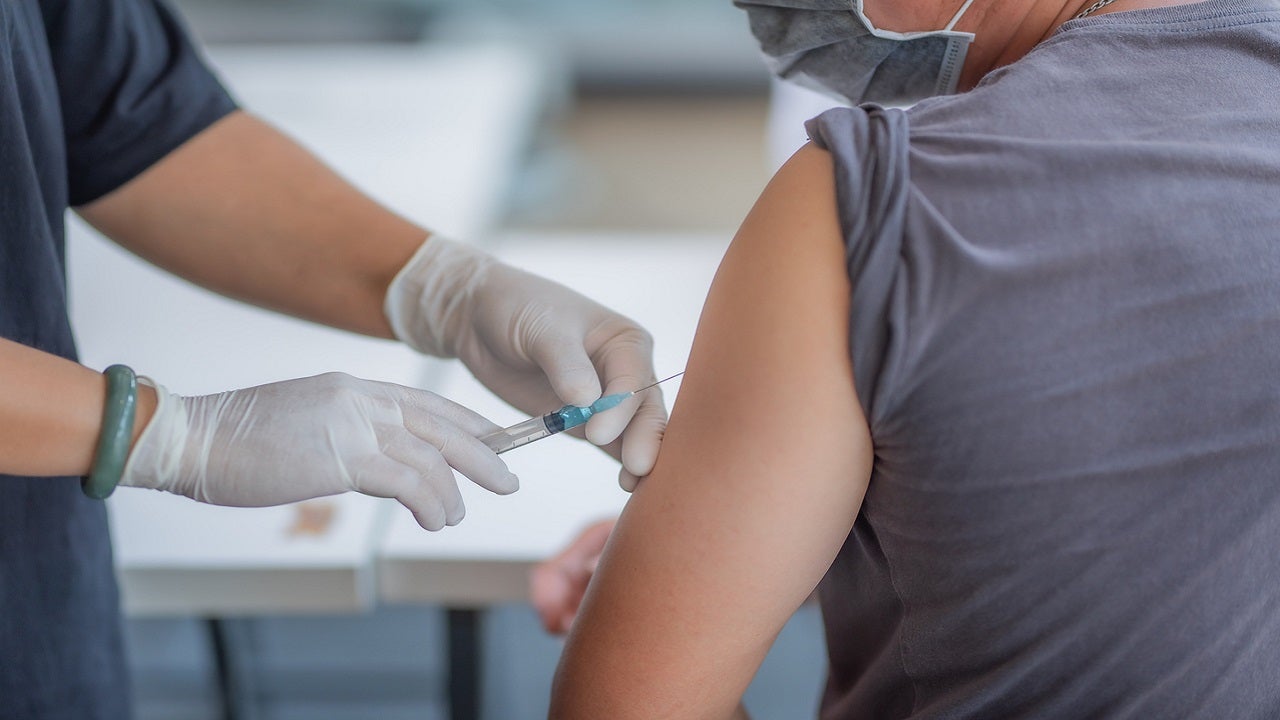
Pfizer-BioNTech seek COVID-19 vaccine authorization for kids in EU
Fox News
Pfizer Inc. and BioNTech have submitted a request to the European drug regulator for the approval of their coronavirus vaccine to be extended to include children 12 to 15 years old, in a move that could offer younger and less at-risk populations in Europe access to the shot for the first time.
BioNTech and Pfizer have previously requested their emergency use authorization with the U.S. Food and Drug Administration also be extended to children 12 to 15 years old. German Health Minister Jens Spahn welcomed the news that the vaccine might soon get the green light for older children.More Related News













