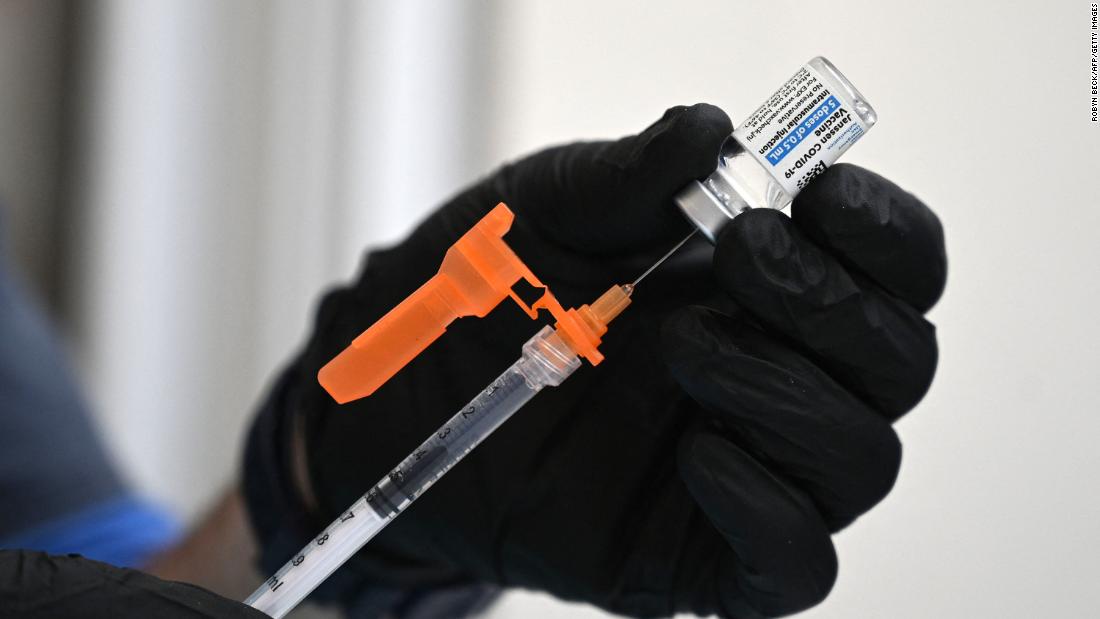
Johnson & Johnson asks FDA to authorize Covid-19 vaccine booster shots
CNN
Johnson & Johnson has asked the FDA to authorize booster shots for its coronavirus vaccine, but has left it up to the FDA and the US Centers for Disease Control and Prevention to decide just who should get their boosters and when.
"We're describing the data to them," Dr. Mathai Mammen, head of global research and development for J&J's vaccine arm, Janssen, told CNN.
"The process is not that we asked for a very specific interval -- we're providing them data and we're going to be presenting to the committee. They'll take all that into consideration when they ultimately decide on an appropriate interval."

More than two decades ago, on January 24, 2004, I landed in Baghdad as a legal adviser, assigned an office in what was then known as the Green Zone. It was raining and cold, and my duffle bag was thrown into a puddle off the C-130 aircraft that had just done a corkscrew dive to reach the runway without risk of ground fire. Young American soldiers greeted me as we piled into a vehicle, sped out of the airport complex and then along a road called the “Highway of Death” due to car bombs and snipers.












