HIV prevention drug lenacapavir approved by FDA as twice-yearly injection
CBSN
The U.S. Food and Drug Administration has approved the drug lenacapavir as a twice-yearly injection to prevent HIV.
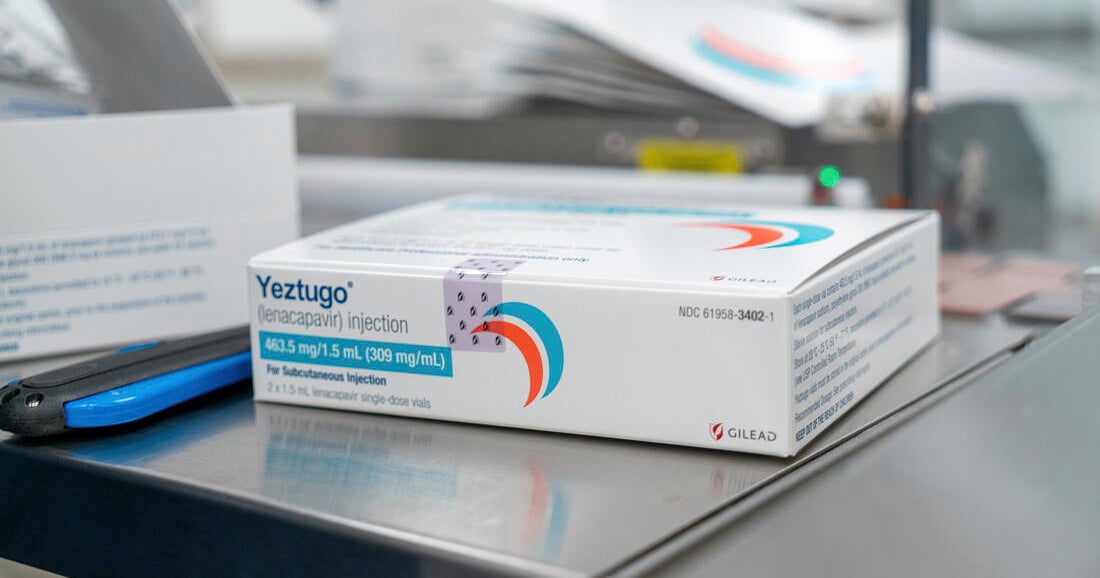
The drug, called Yeztugo from company Gilead Sciences, was approved Wednesday based on data from clinical trials that showed 99.9% of participants who received it remained HIV negative.
Daniel O'Day, Gilead's chairman and chief executive officer, called the approval a "milestone moment in the decades-long fight against HIV."
More Related News
