FDA postpones advisory panel meeting on Pfizer Covid-19 vaccine for younger children
CNN
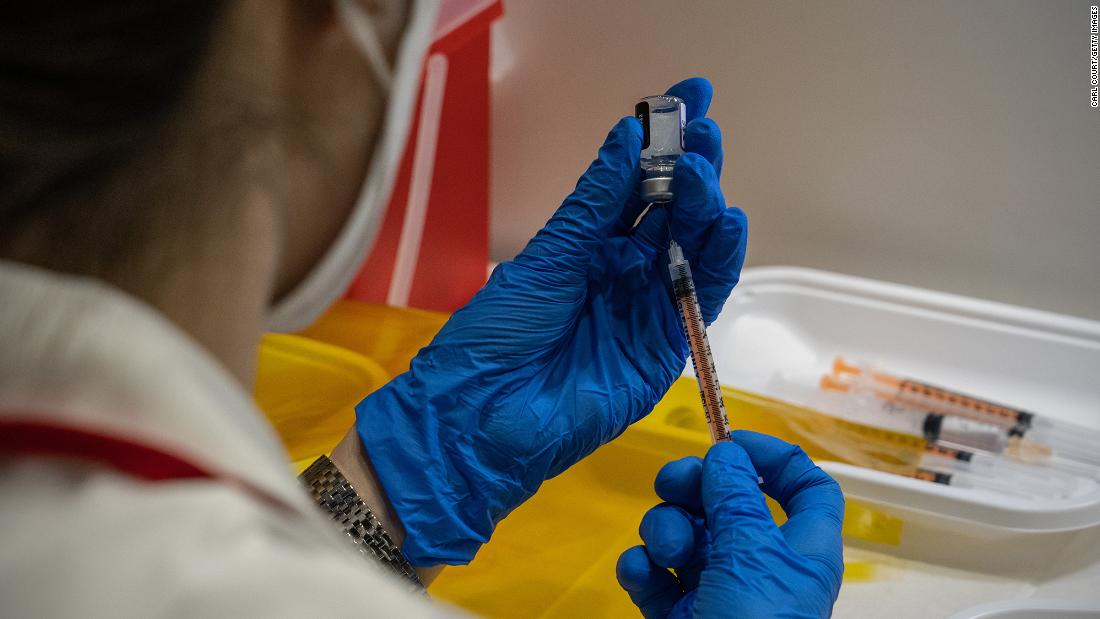
The US Food and Drug Administration announced Friday that it is postponing the meeting of its Vaccines and Related Biological Products Advisory Committee, originally scheduled for February 15, as "new data have recently emerged" regarding Pfizer and BioNTech's emergency use authorization request for their Covid-19 vaccine for children younger than 5.
"This will give the agency time to consider the additional data, allowing for a transparent public discussion as part of our usual scientific and regulatory processes for COVID-19 vaccines. We will provide an update on timing for the advisory committee meeting once we receive additional data on a third dose in this age group from the company's ongoing clinical trial and have an opportunity to complete an updated evaluation," acting FDA Commissioner Dr. Janet Woodcock and Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in a statement.
In a news release Friday, Pfizer said it continues to share data from its child vaccine trial with the FDA. With the surge of the Omicron variant, high rates of infection and illness among children have allowed the company to quickly accumulate the data it needs for the study protocol.

Dos semanas después del derrocamiento de Nicolás Maduro, los ciudadanos venezolanos que viven en diferentes países de la región siguen con atención lo que ocurre en la tierra que los vio nacer. Jimena de la Quintana visitó Gamarra, el emporio comercial más grande de Perú y uno de los más importantes de Latinoamérica, que es fuente de empleo de muchos venezolanos. ¿En qué condiciones regresarían esos migrantes venezolanos a su país? ¿Para ellos es suficiente que Maduro ya no esté en el poder?

The Pentagon has ordered the military command that oversees new recruits’ enlistment to hold off on initial training for people who are HIV-positive and recently enlisted in the military, CNN has learned, saying that a decision on reinstating a Defense Department ban on their joining the military was “expected in the next few weeks.”

The European Union and the Mercosur bloc of South American countries formally signed a long-sought landmark free trade agreement on Saturday, capping more than a quarter-century of torturous negotiations to strengthen commercial ties in the face of rising protectionism and trade tensions around the world.

Judge restricts federal response to Minnesota protests amid outrage over immigration agents’ tactics
Immigration agents carrying out a sweeping operation in Minnesota can’t deploy certain crowd-control measures against peaceful protesters or arrest them, a federal judge ruled Friday. The order follows widespread outrage over a fatal shooting, reports of US citizens getting detained and Minnesotans getting asked for documents for no clear reason.

The smell of wet grass from the recent atmospheric river rains, mud and gasoline wafts through the warm Southern California air as Alec Derpetrossian works the chainsaw with a foreman, Randy Magaña, who helps him guide where to put the blade. Derpetrossian is still learning how to adequately use the large tool.








