FDA delays meeting on COVID-19 vaccine for children under 5 to review more data
CBSN
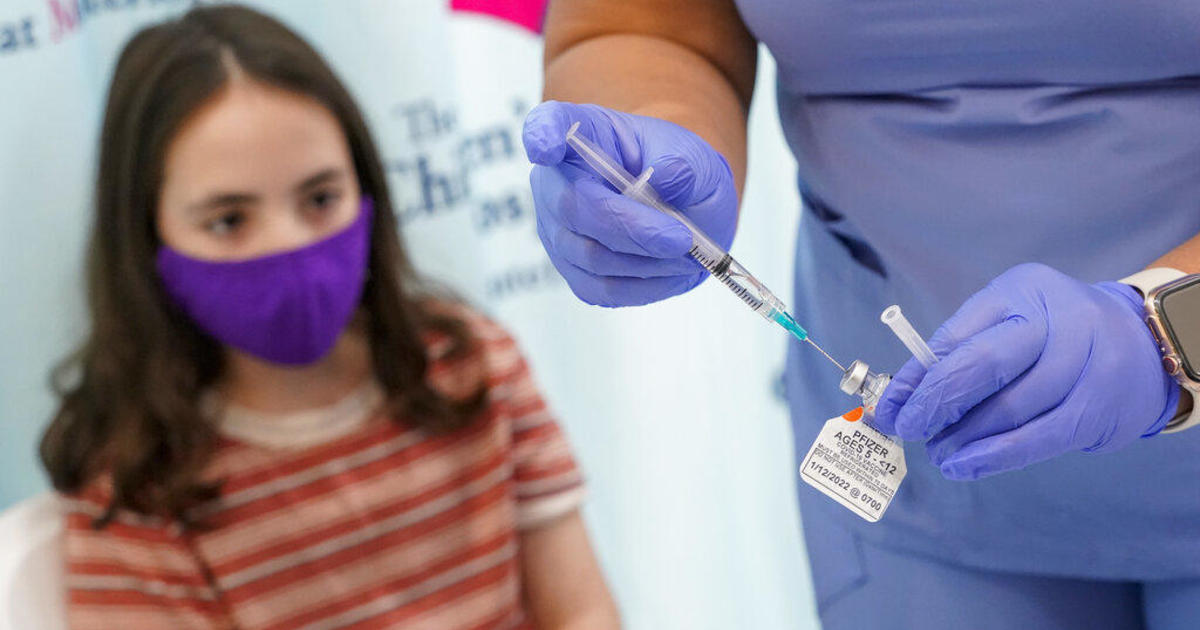
The U.S. Food and Drug Administration has delayed a meeting about a COVID-19 vaccine for children younger than 5 that was originally scheduled to take place next week, raising questions about when they'll be able to get vaccinated against the deadly virus. The FDA said it wants to see more data from Pfizer before proceeding.
Kids younger than 5 are the only age group in the U.S. that cannot yet get the vaccine.
The FDA said it is postponing a meeting by an expert panel — scheduled for February 15 — to give the agency time to consider additional data, "allowing for a transparent public discussion as part of our usual scientific and regulatory processes for COVID-19 vaccines."
More Related News
