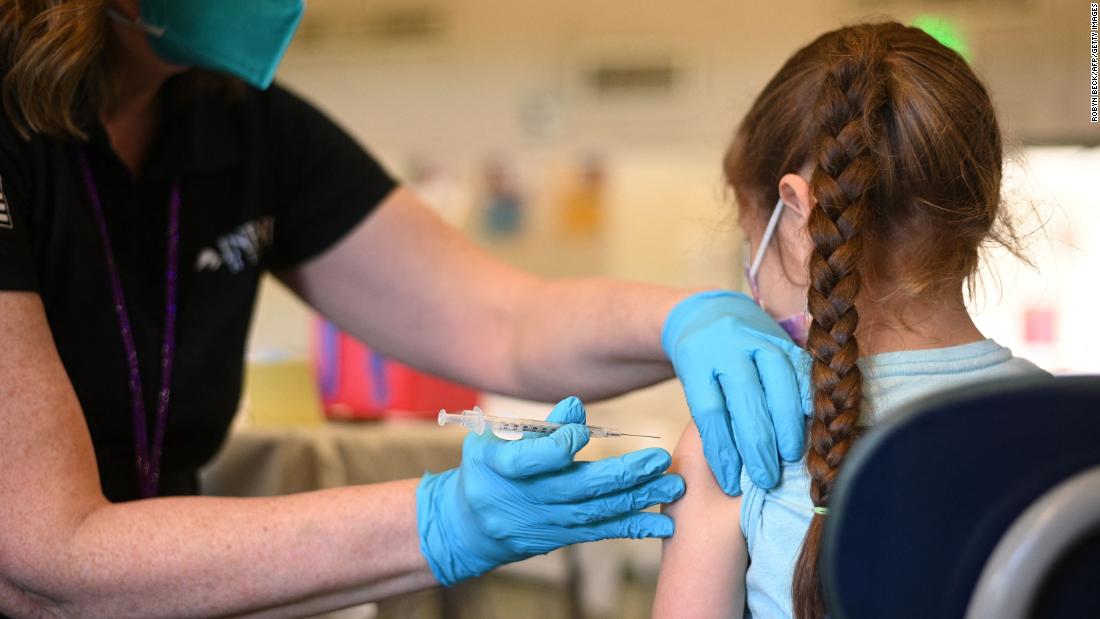
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11
CNN
The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36 times in this age group.
"While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer term effects, even following initially mild disease," FDA Commissioner Dr. Robert Califf said in a news release Tuesday. "The FDA is authorizing the use of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age to provide continued protection against COVID-19.

Hundreds of Border Patrol officers are mobilizing to bolster the president’s crackdown on immigration in snowy Minneapolis, Homeland Security Secretary Kristi Noem said Sunday, as tensions between federal law enforcement and local counterparts flare after an ICE-involved shooting last week left a mother of three dead.

Nationwide outcry over the killing of a Minneapolis woman by an Immigration and Customs Enforcement agent spilled into the streets of cities across the US on Saturday, with protesters demanding the removal of federal immigration authorities from their communities and justice for the slain Renee Good.

Since early December the US Coast Guard and other military branches have boarded and taken control of five oil ships that had previously been sanctioned, all either accused of being in the process of transporting Venezuelan oil or on their way to take on oil that has been subject to US sanctions since President Donald Trump began a pressure campaign against the leadership of the country during his first term.










