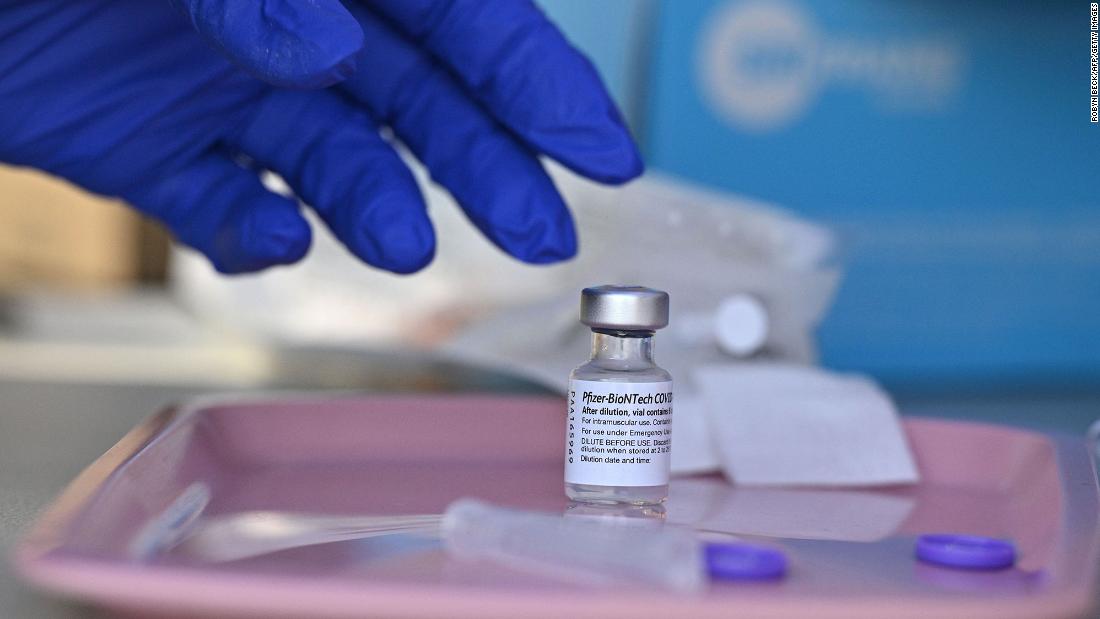
Covid-19 vaccine for 5- to 11-year-olds is safe and shows 'robust' antibody response, Pfizer says
CNN
A Phase 2/3 trial of Pfizer/BioNTech's two-dose Covid-19 vaccine for children ages 5 to 11 showed it is safe and generated a "robust" antibody response.
These are the first such results released for this age group for a US Covid-19 vaccine, and the data has not yet been peer-reviewed or published. Pfizer said it plans to submit to the US Food and Drug Administration for emergency use authorization soon.
The trial included 2,268 participants ages 5 to 11 and used a two-dose regimen of the vaccine administered 21 days apart. This trials used a 10-microgram dose -- smaller than the 30-microgram dose that has been used for those 12 and older.

Secretary of Defense Pete Hegseth announced Monday that the Pentagon is taking administrative action to punish Sen. Mark Kelly, a retired Navy captain, by cutting his retirement pay for participating in a video where he and other Democratic lawmakers reminded US service members of their duty to refuse illegal orders.












